The biological activity of 2-dialkoxyphosphoryl-1,4-dihydrobenzodiazines was analyzed by PASS (Prediction of Activity Spectra for Substances) and toxicity by GUSAR (General Unrestricted Structure-Activity Relationships)
Getting these compounds:
2-Diethoxyphosphoryl-1,4-dihydrobenzodiazine Hydrochloride 1(a). A solution of aldehyde 1a (0.86 g, 4 mmol) in ether (5 ml) was added with stirring to a solution of o-phenylenediamine (0.43 g, 4 mmol) in ether (20 ml) at 0 °С. The reaction mixture was stirred with cooling for 1 h and at room temperature for 2 h. The precipitate of 1(a) was filtered off and recrystallized from ethanol- acetonitrile to give 1.03 g (84 %).
2-Diisopropoxyphosphoryl-1,4-dihydrobenzodiazine Hydrochloride 1(b) was obtained analogously in 87 %.
To determine the potential biological activity, the PASS program was chosen, which is based on an analysis of the structure-activity dependencies. The forecast results are presented to the user in the form of a list of names of probable types of activity with calculated estimates of the probabilities of presence (Pa) and absence of each type of activity (Pi), which have values from 0 to 1. Pa and Pi are presented as estimates of the measure of membership of the substance in the classes of active and inactive compounds respectively. The larger the Pa value for a specific activity and the smaller the Pi value, the greater the chance of detecting this activity in the experiment. Predictions of the biological activity of the compounds are shown in table 1.
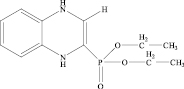
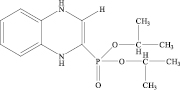
1(а) 1(b)
Table 1
Prediction of biological activity according to the results of the PASS program
1(а)
|
№ |
Pa |
Pi |
Activity |
|
1 |
0,877 |
0,004 |
Antihypertensive |
|
2 |
0,772 |
0,018 |
2-Alpha-N-acetylglucosaminyl transferase inhibitor |
|
3 |
0,714 |
0,009 |
Cutinase Inhibitor |
|
4 |
0,742 |
0,051 |
Aspulvinone Dimethylallyl Transferase Inhibitor |
|
5 |
0,695 |
0,012 |
Acetyl esterase inhibitor |
|
6 |
0,681 |
0,023 |
Pseudolysin Inhibitor |
|
7 |
0,671 |
0,039 |
Sugar phosphatase inhibitor |
|
8 |
0,662 |
0,031 |
5-O- (4-coumaroyl) -D-quinate 3’-monooxygenase inhibitor |
|
9 |
0,655 |
0,029 |
Dehydro-L-Gulonate Decarboxylase Inhibitor |
|
10 |
0,633 |
0,022 |
Thioredoxin Inhibitor |
1(b)
|
№ |
Pa |
Pi |
Activity |
|
1 |
0,918 |
0,004 |
Antihypertensive |
|
2 |
0,866 |
0,004 |
Dehydro-L-Gulonate Decarboxylase Inhibitor |
|
3 |
0,824 |
0,005 |
Supplement Factor D Inhibitor |
|
4 |
0,822 |
0,007 |
Glutamyl Endopeptidase II Inhibitor |
|
5 |
0,804 |
0,005 |
IgA-specific serine endopeptidase inhibitor |
|
6 |
0,798 |
0,012 |
Feruloyl Esterase Inhibitor |
|
7 |
0,764 |
0,004 |
Endopeptidase So Inhibitor |
|
8 |
0,760 |
0,004 |
General pump inhibitor |
|
9 |
0,746 |
0,010 |
2-hydroxy-muconate-semi-aldehyde hydrolase inhibitor |
|
10 |
0,741 |
0,009 |
Acetyl esterase inhibitor |
Table 2
Prediction of acute toxicity in rats using the GUSAR software product
1(а)
|
Rat IP LD50 Log10(mmol/kg) |
Rat IV LD50 log10(mmol/kg) |
Rat Oral LD50 log10(mmol/kg) |
Rat SC LD50 log10(mmol/kg) |
|
0,010 in AD |
-0,886 in AD |
0,185 in AD |
0,037 in AD |
|
Rat IP LD50 (mg/kg) |
Rat IV LD50 (mg/kg) |
Rat Oral LD50 (mg/kg) |
Rat SC LD50 (mg/kg) |
|
274,600 in AD |
34,850 in AD |
411,200 in AD |
292,100 in AD |
1(b)
|
Rat IP LD50 Log10(mmol/kg) |
Rat IV LD50 log10(mmol/kg) |
Rat Oral LD50 log10(mmol/kg) |
Rat SC LD50 log10(mmol/kg) |
|
-0,075 in AD |
-0,967 in AD |
0,450 in AD |
-0,032 in AD |
|
Rat IP LD50 (mg/kg) |
Rat IV LD50 (mg/kg) |
Rat Oral LD50 (mg/kg) |
Rat SC LD50 (mg/kg) |
|
249,300 in AD |
31,960 in AD |
834,300 in AD |
275,200 in AD |
Table 3
Acute classification of rodent toxicity by chemicals under the OECD project using the GUSAR software product
1(а)
|
Rat IP LD50 Classification |
Rat IV LD50 Classification |
Rat Oral LD50 Classification |
Rat SC LD50 Classification |
|
Class 4 in AD |
Class 3 in AD |
Class 4 in AD |
Class 4 in AD |
1(b)
|
Rat IP LD50 Classification |
Rat IV LD50 Classification |
Rat Oral LD50 Classification |
Rat SC LD50 Classification |
|
Class 4 in AD |
Class 3 in AD |
Class 4 in AD |
Class 4 in AD |
As can be seen from table 1, we can say that both compounds exhibit different inhibitory ability.
Next, a forecast of acute toxicity of the test compound was carried out using the software product GUSAR (General Unrestricted Structure-Activity Relationships) presented in tables 2 and 3.
From table 3 we can conclude that the toxicity of both compounds is low, 3-4 hazard class.
Thus, the results obtained in predicting biological activity using the PASS program and the toxicity of the studied compounds using the GUSAR software product allow us to conclude that compound 1 (b) is more active than 1 (a), and the toxicity of both compounds is equal. Therefore, compound 1 (b) has a higher potential activity, and this compound can be prospectively used for further laboratory studies.

