The biological activity of 5-Diethoxyphosphoryl-4-ethoxy-5-ethoxycarbonyl-2-(1-imino)ethylthio-4,5-dihydrothiazole was analyzed by PASS (Prediction of Activity Spectra for Substances) and toxicity by GUSAR (General Unrestricted Structure-Activity Relationships).
Methods of obtaining these substances:
1. 5-Diethoxyphosphoryl-4-ethoxy-5-ethoxycarbonyl-2 – (1-imino)ethylthio-4,5-dihydrothiazole. One solution of thiocyanate acetal (3.83 g, 0.0 l mol) and thioacetamide (0.75 g, 0.01 mol) in absolute acetonitrile or ethanol (30 ml) was heated with a reverse refrigerator for 16 hours. the solvent was removed in vacuum and 10 ml of 3:1 ether-acetone was added to the resulting oil. The yellow crystalline precipitate was filtered and dried to yield 2.31 g (56 %) of the compound.
To determine the potential biological activity chose the program PASS, which is based on the analysis of dependencies structure-activity. Pa and Pi are presented as estimates of the measure of the substance belonging to the classes of active and inactive compounds, respectively. The larger the value ofPa for a particular activity and the smaller the value ofPi, the greater the chance to detect this activity in the experiment. Forecasts of biological activity of compounds are given in table 1.
As can be seen from table 1, it can be said that both compounds exhibit different inhibitory properties.
Further, the forecast of acute toxicity of the studied compound was carried out using the software product GUSAR (General Unrestricted Structure-Activity Relationships) presented in tables 2 and 3.
1. 2.
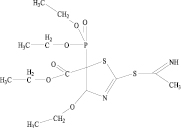
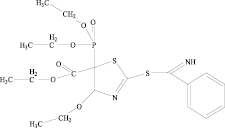
Table 1
Prediction of biological activity based on the results of the PASS program 1
1.
|
Pa |
Pi |
Activity |
|
0,795 |
0,015 |
Mannotetraose inhibitor of 2-alpha-N-acetylglucosaminyltransferase |
|
0,666 |
0,024 |
Supplementfactor d inhibitor |
|
0,607 |
0,009 |
Inhibitorkokolina |
|
0,648 |
0,068 |
Inhibitor of CDP-glycerol-glycerol phosphotransferase |
|
0,613 |
0,039 |
An inhibitor of glutamate-5-polulegendarnaya |
|
0,567 |
0,024 |
Inhibitor of N-acetylneuraminate 7-O (or 9-O) – acetyltransferase |
|
0,579 |
0,054 |
Inhibitor of 5-O – (4-coumaroyl) – D-hinata 3' – monooxygenase |
|
0,584 |
0,059 |
Sugarphosphataseinhibitor |
|
0,503 |
0,004 |
Stimulatorofboneformation |
|
0,507 |
0,020 |
Generalanesthesia |
|
0,541 |
0,060 |
Treatment of acute neurological disorders |
2.
|
Pa |
Pi |
Activity |
|
0,747 |
0,021 |
Mannotetraose inhibitor of 2-alpha-N-acetylglucosaminyltransferase |
|
0,645 |
0,028 |
Supplementfactor d inhibitor |
|
0,598 |
0,032 |
Arginine-2-monooxygenase inhibitor |
|
0,587 |
0,043 |
An inhibitor of glutamate-5-polulegendarnaya |
|
0,543 |
0,014 |
Inhibitorkokolina |
|
0,606 |
0,081 |
Inhibitor of CDP-glycerol-glycerol phosphotransferase |
|
0,515 |
0,019 |
Generalanesthesia |
|
0,556 |
0,061 |
Inhibitor of 5-O – (4-coumaroyl) – D-hinata 3' – monooxygenase |
|
0,495 |
0,005 |
Paraoxonasesubstrate |
|
0,546 |
0,058 |
Treatment of acute neurological disorders |
|
0,525 |
0,048 |
Venomininhibitor AB |
|
0,545 |
0,069 |
Sugarphosphataseinhibitor |
|
0,494 |
0,033 |
Inhibitor of N-acetylneuraminate 7-O (or 9-O) – acetyltransferase |
|
0,456 |
0,005 |
Stimulatorofboneformation |
Table 2
Prediction of acute toxicity in rats using the GUSAR software product 1
1.
|
Rat IP LD50 Log10(mmol/kg) |
Rat IV LD50 log10(mmol/kg) |
Rat Oral LD50 log10(mmol/kg) |
Rat SC LD50 log10(mmol/kg) |
|
-0,539 in AD |
-0,873 in AD |
-0,024 in AD |
-0,329 in AD |
|
Rat IP LD50 (mg/kg) |
Rat IV LD50 (mg/kg) |
Rat Oral LD50 (mg/kg) |
Rat SC LD50 (mg/kg) |
|
119,400 in AD |
55,230 in AD |
390,300 in AD |
193,400 in AD |
2.
|
Rat IP LD50 Log10(mmol/kg) |
Rat IV LD50 log10(mmol/kg) |
Rat Oral LD50 log10(mmol/kg) |
Rat SC LD50 log10(mmol/kg) |
|
-0,311 in AD |
-0,927 in AD |
0,487 in AD |
-0,047 in AD |
|
Rat IP LD50 (mg/kg) |
Rat IV LD50 (mg/kg) |
Rat Oral LD50 (mg/kg) |
Rat SC LD50 (mg/kg) |
|
231,900 in AD |
56,080 in AD |
1457,000 in AD |
425,500 in AD |
Table 3
Acute classification of rodent toxicity by chemicals by OECD project using GUSAR software product 1
1.
|
Rat IP LD50 Classification |
Rat IV LD50 Classification |
RatOral LD50 Classification |
Rat SC LD50 Classification |
|
Class 4 in AD |
Class 4 in AD |
Class 4 in AD |
Class 4 in AD |
2.
|
Rat IP LD50 Classification |
Rat IV LD50 Classification |
RatOral LD50 Classification |
Rat SC LD50 Classification |
|
Class 4 in AD |
Class 4 in AD |
Class 4 in AD |
Class 4 in AD |
From table 3 it can be concluded that the toxicity of both compounds is low, 4 hazard classes.
Thus, the results obtained by predicting the biological activity using the PASS program, and the toxicity of the studied compounds using the GUSAR software product allow us to conclude that compound 1 is more active than 2, and the toxicity of both compounds is equal. Therefore, compound 1 has a higher potential activity, and this compound can be used prospectively for further laboratory studies.

