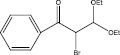
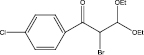
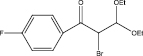
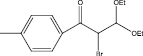
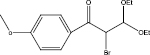
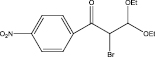
We examined three stages of the synthesis of 2-bromo-3,3-diethoxy-1-phenylpropan-1-one. The obtained α-halocarbonyl compound is interest as an active reagent for the preparation of functionally substituted acyclic and heterocyclic substances. Consider the stages of synthesis.
Stage 1:
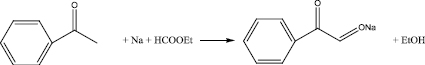
Absolute diethyl ether, sodium metal and absolute alcohol were placed in a three-necked 4-liter flask equipped with a stirrer, a thermometer, a dropping funnel and a reflux condenser with a calcium chloride tube. The reaction mass was cooled to -5 °C and a mixture of ethyl formate and acetophenone was added through a dropping funnel so that the reaction temperature did not exceed 5 °C. After instillation, the reaction mass was stirred for 60 min at 0 °C, and removed to a refrigerator in the cooling mixture overnight. Then it was stirred for three days at room temperature and 90 min at 34 °C. The precipitate was filtered off, washed with ether and dried in vacuo, then triturated in a mortar and dried again.
Stage 2:
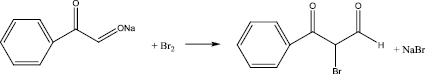
The ground sodium enolate obtained in stage 1 was placed in a three-necked 1-liter flask equipped with a stirrer, a thermometer, a dropping funnel and a reflux condenser with a calcium chloride tube, mixed with dichloromethane. The reaction mass was cooled to -5 °C and bromine was added through a dropping funnel so that the temperature did not exceed 5 °C. Upon completion of the dropping, the reaction mass was stirred to room temperature, and then 90 min at a temperature of 40 °C. The precipitate (sodium bromide) was filtered off under vacuum. The filtrate was removed in the refrigerator in the cooling mixture overnight. The precipitated crystals were filtered off under vacuum, washed with dichloromethane and dried under vacuum, then triturated and dried again.
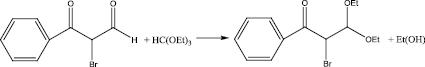
Stage 3
|
№ |
Соединение |
Arginine – 2 – monoxidase inhibitor activity |
|
1 |
|
0,884 |
|
2 |
|
0,816 |
|
3 |
|
0,816 |
|
4 |
|
0,826 |
|
5 |
|
0,739 |
|
6 |
|
0,700 |
The aldehyde obtained in stage 2 was mixed with benzene in a 200 ml flask, under heating, zinc chloride was added to initiate the reaction, then triethylorthoformate was added. Stirred at room temperature for 6 hours. At the end left at room temperature overnight. Triethylorthoformate was evaporated in vacuo, carbon tetrachloride was added, and a fine precipitate formed. It was filtered under vacuum, the filtrate was evaporated and put in the refrigerator overnight.
Range of functionally substituted derivatives of the obtained compound was considered (table). Using the PASS program, an analysis was made of the biological activity of the compounds with respect to the arginine – 2 – monooxidase enzyme. The table shows that all compounds exhibit high activity in relation to this enzyme. Since they are inhibitors of arginine – 2 – monooxidase, the compounds under consideration prevent the destruction of various monoamines, for example, serotonin, dopamine. According to the data obtained, it is seen that there is a decrease in activity in the presence of substituents in the benzene ring, and the activity is the same for chlorine and fluorine substituted [2, 3].